Custom Packs
Customised Procedure Packs
Formatted for Aseptic PharmacyAMD Sterile Transfer Sets & Accessories
A unique AMD service
Many times an aseptic procedure may call for a combination of devices/components which do not exist within AMD’s standard configuration of products. At this point it is worth considering a bespoke approach. In the aseptic preparation and transfer of drug formulations, users of the necessary medical devices may have a ‘wish list’ of the ideal number of components required.
AMD works with users, to create a pack unique to their procedures. The aim of these packs is to support the recognised traditional method of transfer.
The customised procedure pack approach can help support the release of staff time, increase productivity and aid with capacity planning.
This approach can assist by:
- Improve speed of manufacture and efficiency
- Save significant time for recording and checking individual components
- Simplifying the transfer process and reduces variance of the transfer process between operators
- Reduction in use of wipes and spray
- Increased quality assurance
- Ease of accessibility whilst gloved or when working in an isolator
- Reduction in manipulations during the manufacturing process, as many components can be ‘linked’
- Increased protection of the fluid pathway
- Removing all paper from the process
- Having the exact number of devices required for a procedure
- Reduction in waste stream
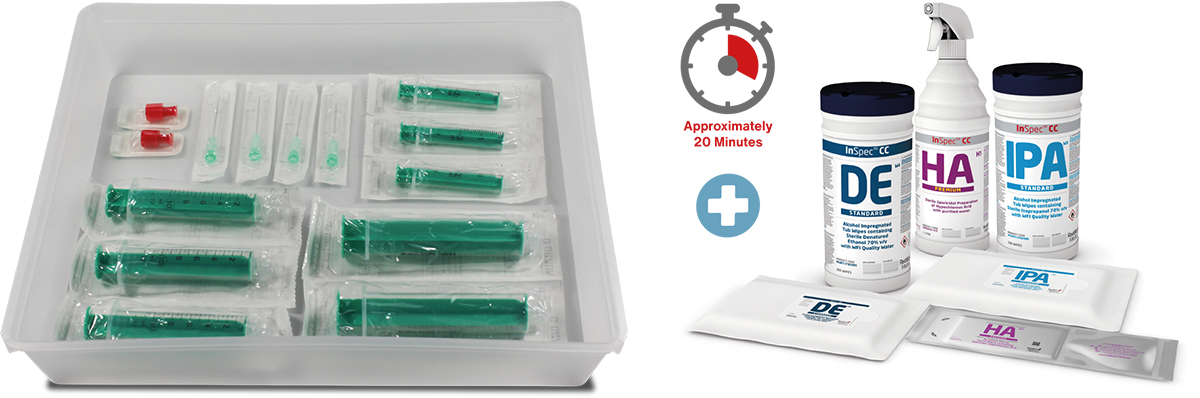
There have been numerous studies that show the savings made and risk reduction by utilising Custom Procedure Packs. Imagine not having to Spray & Wipe each individual component before transferring to the next area.
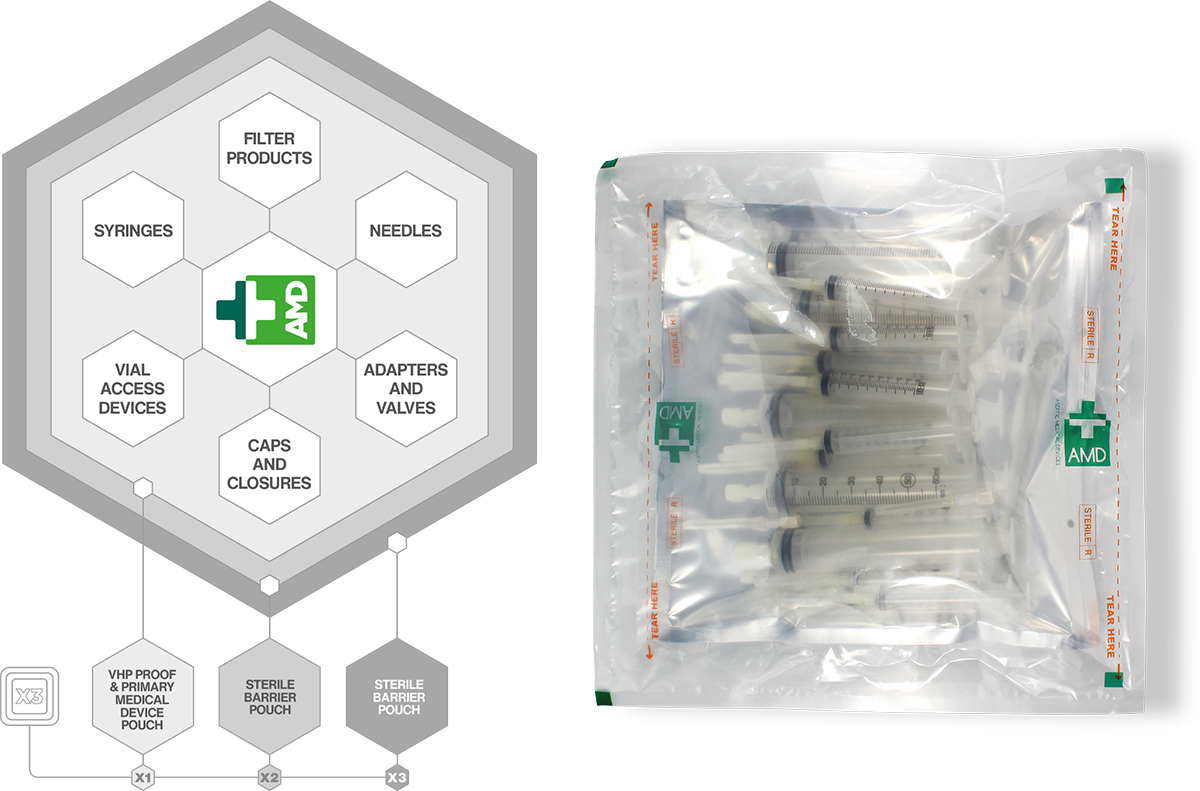
How easy would it be knowing that the exact component and quantity are contained within a Triple Sterile Pouch System. Aseptic Medical Devices has qualified and validated all components that are used within Procedure Packs. This allows the end user to “mix and match” components to suit their requirements.
Added to this we are able to link devices where required meaning less manipulations by the end user and less risk during your production process.
- Qualified and Validated Components
- Triple Sterile Wrapped
- Non-Sterile evaluation sample upon request