Pouches
AMD Pouches
Formatted for Aseptic PharmacyAMD Sterile Transfer Sets & Accessories
Product information
The best packaging solution for all transfer methods.
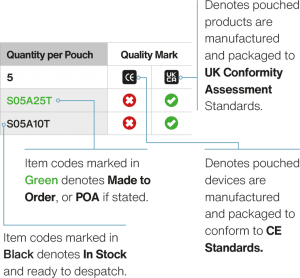
Aseptic Medical Devices’ pouches have been designed with aseptic user requirements in mind. The pouch is constructed from a unique Alcohol, VHP and Sporicide impermeable crystal clear film which is suitable for all transfer methods.
By utilising two variants of this laminate film – a metalised film (PeroxyMet) and visually clear film (PeroxyClear) in the AMD pouch construction, it has allowed the creation of a pouch that makes ‘in process’ inspection simpler and more efficient. Our pouches are manufactured in cleanrooms.
The metalised film half of the pouch provides a high visual background contrast, so that small and semi-translucent devices may easily be inspected through the optically neutral transparent panel.
The pouch incorporates reinforced prepunched holes at the welded end, removing any need for additional steps prior to loading into a VHP gassing port.
Clear, concise print is utilised on the metalised panel, along with pre-cut ‘easy linear tear’ system making opening with the gloved hand straight forward without the need for scissors.
AMD Pouches & Packaging
Products are presented in AMD’s Triple Wrap Sterile System. This comprises of 3 sterile barrier pouches designed to exceed the stringent requirements of today’s transfer process. The secondary and tertiary pouches are fully validated sterile barriers. AMD’s innovative design features have also been carried over on to these layers, with easy tear opening resulting in a simplification of the transfer process with greater quality assurance.
Product is always packed into polythene lined cartons to further separate goods from any sources of particulate and contamination. Each carton is completed with labels and irradiation indicators. Each carton also contains a detachable self adhesive ‘transcription’ labels for each pouch – placed inside the polythene liner.
These mini labels replicate the contents’ product code, its batch number and expiry date, enabling swift physical transfer of critical information to the customer’s own batch documentation should they wish.
Products are then placed into outer shippers, again labelled and with an irradiation indicator.
The Certificate of Conformity and Irradiation for every batch is generated and uploaded to the AMD website where all customers with a login can search and download the appropriate certificate.
If you are a customer and wish to receive a unique login to use this service, please contact: amd@asepticmedical.com.