Hardware
AMD Stainless Steel Needle De-Sheath Re-Sheath Device
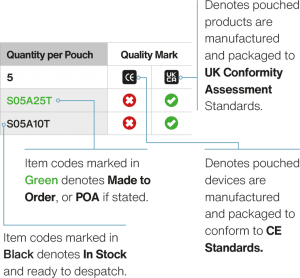
Parts Nos. X01B01A (Standard)
Ordering information
| AMD Stainless Steel 316L | |
|---|---|
| Description | Part No. |
| AMD Stainless steel (316L) de/re sheathing device - standard design (non-sterile) | X01B01A |
Product information
Designed and manufactured by AMD this device is manufactured from 316L stainless steel in once piece. This allows pharmacy operators to more safely remove and replace needle sheaths freeing the operator from close proximity to the needle.
Suitable for left and right handed use. Product is supplied ultrasonically cleanroom cleaned, non-sterile in a polyethylene bag. Registered design No. 4040973.