Filtration
Syringe Filter Devices
Formatted for Aseptic PharmacyAMD Sterile Transfer Sets & Accessories
Ordering information
| Syringe Filters - Capped both ends - VHP pouched and double outer wrapped | Quantity per Pouch | ||||
|---|---|---|---|---|---|
| Description | Pore Size | SmartSite Valve Included | Application | Membrane Material | 5 |
| 28mm Diameter Syringe Filters - Female Luer Lock Inlet, Male Luer Lock Outlet | 0.2μm | No | Sterilising Grade | SFCA* | F05A02A |
| 28mm Diameter Syringe Filters - Female Luer Lock Inlet, Male Luer Lock Outlet | 0.2μm | No | Sterilising Grade | PES** | F05A02P |
| 28mm Diameter Syringe Filters - Female Luer Lock Inlet, Male Luer Lock Outlet | 0.45μm | No | Bioburden Reduction | SFCA* | F05A45A |
| 28mm Diameter Syringe Filters - Female Luer Lock Inlet, Male Luer Lock Outlet | 5.0μm | No | Particulate Removal | CA*** | F05A05A |
| 28mm Diameter Syringe Filters with SmartSite Valve Upstream - Female Luer Lock Inlet, Male Luer Lock Outlet | 5.0μm | Yes | Particulate Removal | CA*** | F05A05B |
* SFCA – Surfactant-free Cellulose Acetate – low protein binding
** PES – Polyethersulfone – low protein binding, high flow
***CA – Cellulose Acetate
Product information
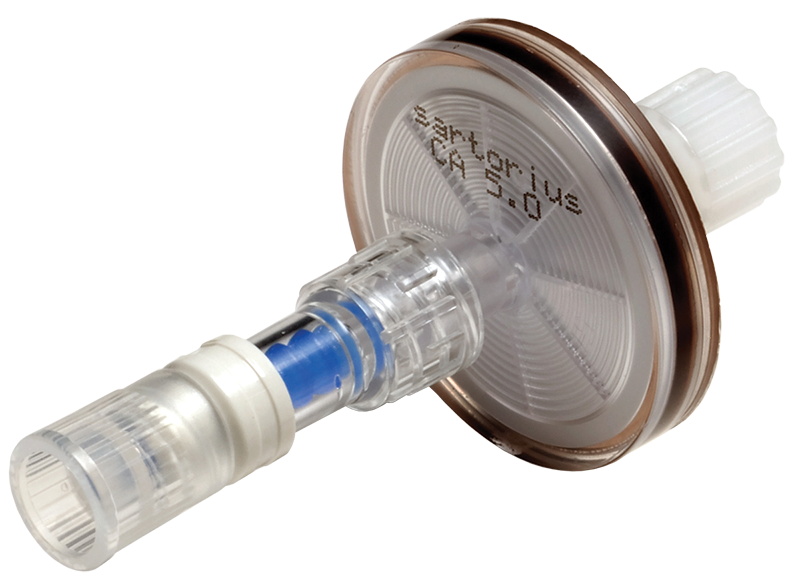
Many parenteral formulations require a final filtration step in their manufacture. To make this step straightforward and rapid, AMD have qualified Sartorius syringe filtration devices both on their own and also prelinked to the BD SmartSite™ valve.
The BD SmartSite™ needle free 2-way, non-drip valve works with standard Luer Lock syringes. The SmartSite™ needlefree valve enables multiple connections and when disconnected from the syringe can withhold 45 psi of back pressure. Both ends of the device(s) are protected by caps.
Filter pore sizes are made available in 0.2 micron for sterilisation of fluids, 0.45 micron for bioburden management and 5 micron for particulate control.
The surfactant free cellulose acetate (SFCA) membrane is suitable for all applications including biological therapeutics (low protein binding).
The Polyethersulphone (PES) membrane is also suitable for low protein binding applications and offers a higher flow rate. Pre-testing is recommended to establish protein binding levels under dynamic filtration conditions.
Aseptic Production Formatting
Each device is fitted with a pin-less Luer Lock cap to protect the surface of the SmartSite™ simplifying aseptic handling and manipulation.